SCHOOL OF MEDICINE
SMSIG
Blog
Welcome to our blog dedicated to aspiring surgeons! Here, students can share their research, discover new topics, and valuable information that will help shape their future careers in surgery.
Join us in creating a community where knowledge and passion for medicine thrive. Let's enhance our skills and prepare for a successful journey in the medical field together!
Laparoscopic Sentinel Lymph Node Mapping with Indocyanine Green for Endometrial Cancer Staging
By: Cameron Rotbart, MS4
10.27.2025

Abstract
Systematic pelvic and para-aortic lymphadenectomy has traditionally been performed for staging in endometrial cancer; however, it is associated with significant morbidity without offering a clear survival benefit. Sentinel lymph node (SLN) mapping with indocyanine green (ICG) and near-infrared (NIR) fluorescence provides accurate nodal assessment while minimizing complications. This technique paper describes a reproducible laparoscopic technique for ICG-guided SLN mapping and summarizes supporting clinical evidence, including learning curve considerations and applicability in molecularly high-risk subgroups. This report underscores the increasing importance of SLN mapping as a minimally invasive technique that maintains oncologic accuracy while reducing the morbidity associated with full lymphadenectomy.
Introduction
Accurate lymph node assessment is essential for staging and treatment planning in endometrial cancer. Historically, systematic pelvic and para-aortic lymphadenectomy, which is when bilateral lymphatic tissue along major blood vessels is removed, was considered standard practice. However, the Efficacy of Systematic Pelvic Lymphadenectomy in Endometrial Cancer (MRC ASTEC) trial demonstrated no overall survival or recurrence-free survival benefit from routine lymphadenectomy in early-stage disease while increasing operative morbidity [1]. While previous randomized trials demonstrated no survival advantage for systematic lymphadenectomy in predominantly low- and intermediate-risk endometrial cancer, the ongoing Endometrial Cancer Lymphadenectomy Trial (ECLAT) aims to clarify its role in high-risk stage I–II disease, with results still pending [2]. This highlights the need for minimally invasive strategies that maintain staging accuracy while reducing morbidity. Laparoscopic SLN mapping using ICG has emerged as one such approach, with studies showing that surgeons achieve consistent bilateral detection after approximately 30 cases, indicating a feasible learning curve and reliable nodal assessment [3].
SLN mapping targets the first draining nodes. These are the nodes that are most likely to harbor metastases [4]. Prior literature has demonstrated that SLN mapping achieves oncologic outcomes comparable to those of complete lymphadenectomy, with the benefit of significantly reduced complications, including nerve injury, vascular injury, lymphedema, increased blood loss, and the risk of infection [4]. Furthermore, SLN ultra-staging enhances detection of low-volume metastases, thereby improving staging accuracy, most notably in patients with high-grade or molecularly high-risk subtypes [5].
Among available visual tracers, ICG in combination with NIR has demonstrated superior bilateral detection rates and rapid real-time visualization compared with blue dyes or radiocolloids [2,6]. Adverse reactions to ICG are rare and are generally mild when they occur [7]. Recent prospective analyses confirm the reproducibility and safety of ICG-guided SLN mapping across several institutions, including minimally invasive approaches such as laparoscopic and Vaginal Natural Orifice Transluminal Endoscopic Surgery (V-NOTES) techniques [3,5].
Learning curve considerations are critical when it comes to this intricate procedure. Approximately 30 procedures are required to achieve ≥ 75% bilateral mapping success, while retrieval of nodes containing lymphatic tissue is achieved in as few as 6 successful cases [5]. Emerging evidence also supports efficacy in high-risk histology, pathology, and molecular subgroups, such p53 abnormal and DNA mismatch repair deficient, providing prognostic information to guide supplemental therapy. Standardization of injection sites, tracer volumes, and ultra-staging protocols remains important to optimize detection and reduce variability [3,4]. This technique aims to provide a reproducible, evidence-based approach that enhances accuracy, minimizes morbidity, and broadens the applicability of SLN mapping across diverse clinical settings.
SURGICAL TECHNIQUE
1. Patient Preparation and Positioning
The proper patient preparation is under general anesthesia, in the dorsal lithotomy and Trendelenburg positioning. A four- or five-port laparoscopic setup is utilized. The endoscope is placed at the level of the umbilicus. A 10-12 mm trocar is then placed in the left lower quadrant, and a 5mm port is placed in the right lower quadrant. Both are positioned lateral to the rectus abdominis muscles and a few centimeters above the anterior superior iliac spine. An accessory port is optional on the left side at a superior-medial position. The use of a uterine manipulator is determined by the clinical judgement of the operating physician.
Figure 1. Schematic of port placement for robotic-assisted staging of endometrial cancer,
similar to that used in laparoscopic sentinel lymph node mapping [9].
2. Cervical Injection of Indocyanine Green
ICG 1.25 mg/mL is used, 1 mL per quadrant; both superficial and deep injections are made at the 3 and 9 o’clock positions. It is common clinical practice to then massage the cervix for increased lymphatic uptake of ICG.
Figure 2. Cervical ICG injection [10]
3. Intraoperative Mapping
About 5-15 minutes after the cervical injection of ICG, near-infrared imaging is activated, depicting fluorescent lymphatic channels to the SLNs. The operating physician then follows the fluorescent lymphatic channels to locate the SLNs, typically in the obturator, external iliac, and occasionally, the common iliac or para-aortic regions.
Figure 3. Intraoperative identification of lymph nodes [12].
4. Lymph Node Dissection and Retrieval
For proper node dissection, the peritoneum that overlies the lymphatic channel is incised, and through blunt dissection, the excision of fluorescent nodes can be performed while preserving the hypogastric nerves and pelvic splanchnic branches. Once excised, the nodes are placed in separate containers for ultra-staging. Pathologic ultra-staging of SLNs is essential to identify isolated tumor cells or micrometastases that may guide adjuvant therapy [1–5]
Figure 4. Excised SLNs: a) Lymph node, b) Fluorescence visualization of the lymph node, and c) overlay of the lymph node illustrating the fluorescence activation [11].
5. Mapping Failure Management
In cases where SLN mapping fails, a side-specific lymphadenectomy should be performed. The reason for failure must be documented, as it is critical for accurate staging and should be interpreted in the context of the patient’s molecular risk profile.
6. Closure and Post Operative Course
After SLN removal, it is common to also perform a total laparoscopic hysterectomy with bilateral salpingo-oophrectomy. For closure, it is essential to ensure adequate hemostasis, remove the trocars from the abdomen and pelvis, as well as perform fascial and port site closure. Similar to that of a minimally invasive hysterectomy pathway, postoperative care involves early ambulation, rapid diet advancement, and discharge often within 24 hours. Adequate pain management, as well as confirming urinary function, are crucial in the postoperative period. Prophylactic anticoagulation and compression devices are recommended according to institutional venous-thromboembolism protocols. Patients are monitored for urinary retention, fever, and signs of lymphatic leak or infection [1–4].
Discussion
Advantages
Laparoscopic SLN mapping with ICG offers several advantages. One major benefit is the lower perioperative morbidity when compared with full pelvic and para-aortic lymphadenectomy. This technique reduces blood loss, operative time, and the risk of chronic lymphedema or lymphocyst formation [1–4]. It is additionally beneficial due to its high staging accuracy, with bilateral detection rates typically exceeding 70%, and sensitive ultra-staging properties [2–6].
An important advantage of this technique is its broad applicability with reliable results, demonstrated across different histologic subtypes and molecular risk groups, including p53-abnormal and MMR-deficient tumors [5,8]. Lastly, the training curve should not be ignored; in one study observing seven surgeons, stable bilateral mapping was consistently achieved after approximately 30 cases [3].
Limitations
The limitations of this method of staging include the specialized requirements, such as near-infrared imaging equipment and formal training are essential, which may limit access in resource-constrained regions [4,5]. Mapping failure is also a potential risk. About 10–15% of procedures require side-specific lymphadenectomy when drainage cannot be visualized [2–5]. Another notable limitation is the risk of potential false negatives due to anomalous lymphatic anatomy or technical factors, which can, on occasion, lead to missed disease. Ultimately, the cost must also be considered; the upfront expenses for ICG and imaging platforms may be higher, though often offset by shorter recovery.
Complications
Possible complications of this procedure include vascular or ureteral injury during retroperitoneal dissection, rare allergic reactions to ICG (estimated at <0.1%) [7], and dye extravasation at the cervical injection site. It should be noted that transient neuropathy from prolonged Trendelenburg positioning, port-site infection or hernia, and deep venous thrombosis may occur, mirroring those of other laparoscopic procedures [3,4]. Lymphocyst formation and lower-limb lymphedema occur less frequently than after systematic lymphadenectomy, approximately less than <3% compared to 15–20% with full nodal dissection [2,4].
Prompt recognition of complications, particularly bleeding or thermal injury, is critical in these types of surgeries. In cases of mapping failure as well as intraoperative suspicion of nodal metastasis, it is recommended to convert to side-specific or full lymphadenectomy to avoid under-staging [2–4].
Conclusion
ICG-guided laparoscopic SLN mapping provides accurate nodal staging with markedly reduced morbidity compared to systematic lymphadenectomy. When performed by trained teams using standardized protocols in combination with ultra-staging, it enhances diagnostic accuracy and informs adjuvant therapy decisions across both low- and high-risk disease while supporting a faster recovery and a better quality of life.
Acknowledgements
Figures 1-4 were included solely for the purpose of illustrating the procedural steps described in this surgical technique. All rights remain with the original copyright holders.
References
-
Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK; ASTEC study group. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136. doi:10.1016/S0140-6736(08)61766-3
-
Emons G, Kim JW, Weide K, de Gregorio N, Wimberger P, Trillsch F, Gabriel B, Denschlag D, Kommoss S, Aydogdu M, Papathemelis T, Gropp-Meier M, Muallem MZ, Kühn C, Müller A, Frank M, Weigel M, Bronger H, Lampe B, Rau J, Schade-Brittinger C, Harter P. Endometrial Cancer Lymphadenectomy Trial (ECLAT): pelvic and para-aortic lymphadenectomy in patients with stage I or II endometrial cancer with high risk of recurrence (AGO-OP.6). Int J Gynecol Cancer. 2021;31(7):1075–1079. doi:10.1136/ijgc-2021-002703
-
Gedgaudaite M, Paskauskas S, Bartusevicius A, Celiesiute J, Svedas E, Vaitkiene D, Drejeriene E, Inciura A, Gaurilcikas A. Laparoscopic sentinel lymph node mapping with indocyanine green in endometrial cancer: surgeon's learning curve (cumulative sum analysis). Int J Gynecol Cancer. 2023;33(4):521–527. doi:10.1136/ijgc-2022-004033
-
Buda A, Bussi B, Di Martino G, Di Lorenzo P, Palazzi S, Grassi T, Milani R. Sentinel lymph node mapping with near-infrared fluorescent imaging using indocyanine green: a new tool for laparoscopic platform in patients with endometrial and cervical cancer. J Minim Invasive Gynecol. 2016;23(2):265–269. doi:10.1016/j.jmig.2015.09.022
-
Bogani G, Murgia F, Ditto A, Raspagliesi F. Sentinel node mapping vs. lymphadenectomy in endometrial cancer: a systematic review and meta-analysis. Gynecol Oncol. 2019;153(3):676–683. doi:10.1016/j.ygyno.2019.03.254
-
Buda A, Di Martino G, Vecchione F, Bussi B, Dell'Anna T, Palazzi S, Cantù MG, Marchette MD, Milani R. Optimizing strategies for sentinel lymph node mapping in early-stage cervical and endometrial cancer: comparison of real-time fluorescence with indocyanine green and methylene blue. Int J Gynecol Cancer. 2015;25(8):1513–1518. doi:10.1097/IGC.0000000000000526
-
Hope-Ross M, Yannuzzi LA, Gragoudas ES, Guyer DR, Slakter JS, Sorenson JA, Krupsky S, Orlock DA, Puliafito CA. Adverse reactions due to indocyanine green. Ophthalmology. 1994;101(3):529–533. doi:10.1016/S0161-6420(94)31303-0
-
Ye L, Li S, Lu W, He Q, Li Y, Li B, Wang X, Yan Q, Wan X. A prospective study of sentinel lymph node mapping for endometrial cancer: is it effective in high-risk subtypes? Oncologist. 2019;24(12):e1381–e1387. doi:10.1634/theoncologist.2019-0113
-
Malgorzata, L., Verena, B.-S., Kati, H., Achim, S., Simone, M., & Christhardt, K. (2010). Surgical treatment of endometrial cancer. Journal of Cancer Therapy, 01(04), 181–191. https://doi.org/10.4236/jct.2010.14028
-
Li, Y., He, L., Hou, Q., Zhang, Q., Gu, D., & Lin, Y. (2023). Retroperitoneal sentinel lymph node biopsy by transvaginal natural orifice transluminal endoscopic surgery in early stage endometrial cancer: A video. Gynecology and Pelvic Medicine, 6, 25–25. https://doi.org/10.21037/gpm-23-4
-
Crane, L. M., Themelis, G., Pleijhuis, R. G., Harlaar, N. J., Sarantopoulos, A., Arts, H. J., van der Zee, A. G., Ntziachristos, V., & van Dam, G. M. (2011). Intraoperative multispectral fluorescence imaging for the detection of the sentinel lymph node in cervical cancer: a novel concept. Molecular imaging and biology, 13(5), 1043–1049. https://doi.org/10.1007/s11307-010-0425-7
-
Somashekhar, S. P., Arvind, R., Kumar, C. R., Ahuja, V., & Ashwin, K. R. (2021). Sentinel node mapping using indocyanine green and near-infrared fluorescence imaging technology for endometrial cancer: A prospective study using a surgical algorithm in Indian patients. Journal of minimal access surgery, 17(4), 479–485. https://doi.org/10.4103/jmas.JMAS_154_20




Reconstructing Cartilage: The Future of Osteoarthritis Management
By: Liz Powell, MD & Benson Gladison, MS3
7.7.2025

Introduction
Osteoarthritis (OA) affects approximately 32.5 million adults in the United States alone [1]. Serving as the most common form of arthritis, OA commonly affects individuals between the ages of 55 and 64 [1]. OA can lead to severe pain, psychological disorders, restrictions on activities, and decreased quality of life. Management of the disease currently involves pharmacological or surgical treatments [2]. Stem cell therapy offers a promising alternative due to the cells’ ability to differentiate and generate new chondrocytes. Additionally, scaffolds provide the necessary structure for cellular adhesion, nutrient diffusion, and tissue development. While these treatments can offer some relief to patients, further investigation into innovative cartilage regenerative techniques is warranted.
Articular Cartilage
Articular cartilage is a type of fibrocartilage commonly found in synovial joints [3]. This cartilage contains both type I and type II collagen, with type I collagen providing additional strength and durability [3]. Collagen plays a crucial role in promoting elasticity and structural integrity within the joint. Additionally, articular cartilage has a high water content, allowing it to function as both a shock absorber and joint lubricant, while also maintaining an avascular environment that depends on diffusion for nutrient exchange and waste removal [3,4]. A lack of direct blood supply contributes to the cartilage’s limited self-healing capacity, making it susceptible to degeneration and injury. With time, mechanical stress, aging, and trauma can result in cartilage deterioration, leading to conditions such as OA [3].
Cartilage is produced and maintained by chondrocytes, which play a vital role in preserving joint integrity and health through the extracellular matrix (ECM) [3,5]. The ECM contains essential components such as water, collagen, proteoglycans, and non-collagenous proteins, which protect against injury and serve as mechanical signal transducers within cells [4,6]. The ECM is mainly constructed from water, promoting extensive frictional characteristics [4].
Over time, cartilage undergoes remodeling due to degradation. Aging leads to diminished chondrocyte function, resulting in reduced cartilage maintenance and repair [6]. Significant cartilage degeneration, pain, and dysfunction are primary contributors to OA. Cartilage damage most commonly results from injury, overuse, or degeneration [3]. Young patients are more prone to sports-related injuries, whereas older patients are more commonly affected by wear and tear and age-related degeneration [3].
Defining Osteoarthritis
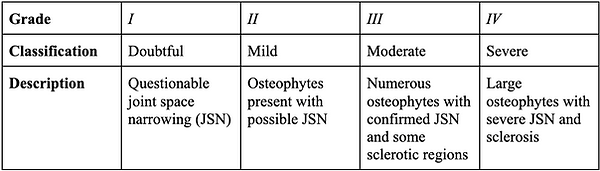
Osteoarthritis presents in numerous fashions—pathological, radiographic, and clinical [7]. Radiographic OA is the ‘standard’ for defining the disease. The Kellgren-Lawrence (K/L) system is used to grade radiographic OA [7]. This classification evaluates OA on a scale from 0 to 4, with Grade ≥2 suggesting the presence of an osteophyte. Further advanced grades are based on features such as joint space narrowing, sclerosis, cyst formation, and deformity [7, Table 1]. Additionally, more sensitive imaging, such as magnetic resonance imaging (MRI), can help identify structural variations in joints [7]. Current research suggests further investigation into the effectiveness of MRI in identifying potential disease-modifying interventions faster than standard radiography. Meanwhile, not all individuals with radiographic OA experience clinical symptoms.
Table 1: Kellgren-Lawrence (K/L) grading system for radiographic OA
Householder et al. recognize knee OA as a deficiency of articular cartilage at the medial and lateral condyles of the femur, tibial plateau, and patellofemoral facet [8]. Symptoms present as chronic pain, joint inflammation, and restricted mobility [8]. The exacerbated pain then contributes to decreased joint function and, eventually, leads to physical disability.
Risk Factors
Radiographic OA
Risk factors for radiographic osteoarthritis are multifactorial [7]. Both systemic and local causes contribute to disease development. Systemic factors include, but are not limited to, age, sex, ethnicity, nutrition, and bone density. Meanwhile, local causes are recognized as obesity, a history of trauma, and mechanical factors like muscle weakness [7].
Systemic Causes
Age
Age is the most prominent contributing risk factor for all joints [7]. In patients over the age of 60, radiographic knee OA occurs in over 30% of patients, although not all may note pain symptoms [7]. With age, cartilage degenerates and chondrocyte function declines. Along with mechanical stress, chronic inflammation can alter the subchondral bone metabolism, contributing to progressive OA signs and symptoms [7].
Sex
Further, there is a greater incidence and severity of disease among women compared to men. There is a known increase in OA around menopause in women, which highlights some theories that hormones play a role in the development of OA [7]. For instance, a study investigating patients on hormone replacement therapy displayed a 15% decrease in the need for a total hip or knee arthroplasty compared to women not taking supplemental hormones [9].
Ethnicity
Nelson et al. studied OA prevalence across racial groups, finding comparable hip OA rates between African American (AA) and white women (23% vs. 22%) but slightly higher rates in AA men than white men (21% vs. 17%) [10]. Radiographic OA findings, such as joint space narrowing and osteophytes, are also more common in AAs [7]. Additionally, anatomical differences in femoral and acetabular structures among racial groups warrant further investigation [7].
Similarly, AAs exhibit higher rates of radiographic and symptomatic knee OA than non-Hispanic whites, leading to greater pain and severity scores [11]. Callahan et al. reported that AAs are 50–65% more likely to develop knee OA than whites [12]. Interestingly, Chinese women have a 45% higher prevalence of knee OA than white women, though no significant difference is observed between Chinese and white men [12].
Genetics
Studies have investigated the potential genetic component of OA. Both twin and familial studies have determined heritable components of OA, estimating a 50-65% greater genetic relationship to hand and hip OA [7]. Congenital disorders such as Legg-Calve-Perthes disease, Slipped Capital Femoral Epiphysis, or congenital subluxation have a known relationship with hip OA later in life [7].
Diet
Vitamin D deficiency is one of OA's most notable nutritional risk factors [7]. Depleted levels of vitamin D can lead to fragile bones. McAlindon et al. examined participants in the Framingham Heart Study to evaluate whether serum vitamin D levels and dietary intake could predict the incidence and progression of knee OA [13]. The study determined that the lowest (<27 ng/mL) and middle (27-33 ng/mL) tertiles of serum 25-hydroxyvitamin D have a three-fold increased risk of knee OA compared to subjects in the highest tertile (>33 ng/mL) [13]. Regarding radiographic OA, Lane et al. noted that decreased 25-vitamin D is possibly related to changes found in hip OA imaging, such as joint space narrowing [14]. Nonetheless, there is limited data on the effects of vitamin deficiencies on OA, suggesting further research needs to be conducted to investigate the effects of vitamin D and additional vitamins on OA.
Local Causes
Obesity
Patients with high BMIs have a significant risk of developing OA, especially knee OA. Women who had lost about ten pounds in the Framingham Knee Osteoarthritis Study had approximately a 50% reduced risk of symptomatic knee OA development [15]. The researchers also indicated a clear connection between weight loss and decreased risk of radiographic knee OA [15]. On the other hand, when exploring hip OA risks, patient weight and disease development have no clear relationship [7].
Injury
Knee injury is another prominent risk factor for OA. Common examples of injury include meniscal tears, ACL injuries, and trans-articular fractures [7].
Mechanical Factors
The relationship between muscle strength and OA is an extremely complicated topic of study and is not fully understood. Data suggests that muscle weakness and atrophy are connected to knee OA [7]. Slemenda et al. concluded that patients with asymptomatic radiographic knee OA without muscle atrophy, but with quadriceps weakness, had an increased risk of developing symptomatic knee OA [16].
Discussion
Current Guidelines for Osteoarthritis Management
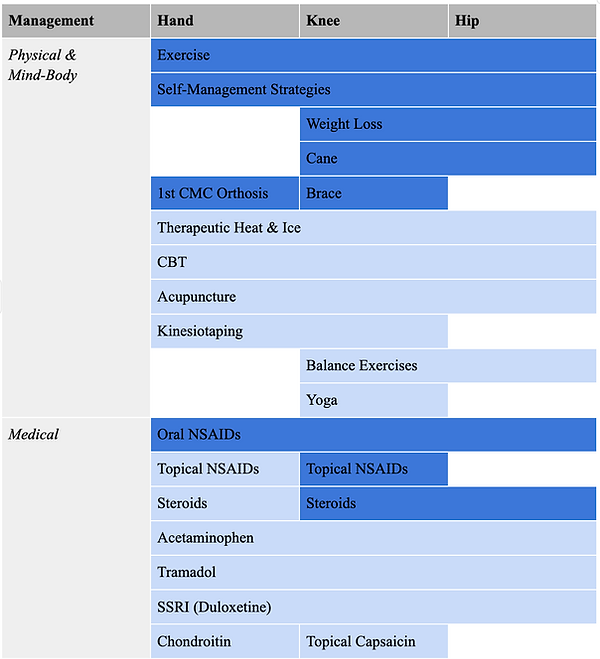
Although there is a significant disparity in OA prevalence, an effective treatment for the disease remains lacking [17]. As the disease is influenced by multiple factors, its treatment approach must also be multifaceted. The American College of Rheumatology (ACR) and the Arthritis Foundation collaborated to develop the 2019 guidelines for managing OA of the hand, knee, and hip [2, Table 1]. When approaching OA treatment, there are two key options: surgical or non-surgical [17]. Regarding the non-surgical approaches, there are strong recommendations for exercise and weight loss in overweight or obese patients with hip or knee OA [17]. Additional knee-specific recommendations include topical NSAIDs, intra-articular injections, and tibiofemoral bracing [8,17]. NSAIDs play a crucial role in reducing inflammation and managing OA symptoms [3]. The first-line pharmacologic agent for managing OA is Tylenol [17]. NSAIDs and COX-2 inhibitors fall under second-line treatment [17]. Topical medications can be used in conjunction with oral treatments to offer more relief. Not to mention, injections, especially those that contain hyaluronic acid (HA), can restore the joint’s lubrication process [17]. It is important to note that the medical management component of OA, specifically injections, does not delay the progression of the disease [8]. Thus, the focus of treatment has shifted to the biomechanisms of cartilage deterioration.
Table 1: Physical and medical approaches to managing OA [2].
Dark blue represents strongly recommended techniques. Light blue reflects conditionally recommended strategies.
Conditional recommendations for OA include yoga, cognitive behavioral therapy (CBT), and self-management strategies [2]. Physical therapy has been shown to improve joint strength and flexibility, while balance training has demonstrated promising outcomes for knee and hip OA [3]. Additionally, orthoses have been particularly effective in managing hand OA [2].
The current standard of surgical interventions for osteochondral defects includes marrow stimulation techniques, autologous chondrocyte implantation, and osteochondral grafting [3,18]. These current treatment options often result in the formation of both type I & II collagen-forming fibrocartilage, whose strength and resilience are lower compared to articular cartilage. Furthermore, they are only applicable for smaller defects, and total joint replacement remains the definitive treatment of OA with larger cartilage defects [18]. Several types of cells, such as chondrocytes or pluripotent cell types that can differentiate into chondrocytes, such as mesenchymal, embryonic, bone marrow, or adipose-derived stem cells, can be potentially used to stimulate cartilage regeneration and maintenance when applied alongside the proper scaffold, such as collagen and proteoglycans [3,18].
Techniques Based On Cell Type
Chondrocytes
The technique of autologous chondrocyte implantation (ACI) was employed initially for cell-based therapy to repair cartilage. In this technique, healthy chondrocytes are harvested from a non-weight-bearing portion of the joint in the initial procedure and then cultured in vitro for 14 to 21 days [19]. The cultured chondrocytes were then injected into the defect, which was covered with a periosteal flap, harvested from the proximal tibia, in a suspension fluid containing collagen [19]. Fibrin glue is then used to seal the affected area. Promising results were seen for this technique, with both pain and swelling reduced and knee locking eliminated [19]. However, ACI comes with the risk of morbidity at the donor site as both the collagen and periosteum are harvested and later implanted, contributing to two separate surgeries [4,18].
The collagen-covered ACI (CACI) is the improved second generation of ACI, which reduced the risk of periosteal compromise by using collagen to cover the defective site [18]. Further advancements led to the third generation of ACI, which uses biomaterial scaffolds as the base on which harvested chondrocytes are attached and later inserted at the lesion site [18]. This new technique was termed matrix-assisted ACI (MACI) [18]. The use of MACI prevents the chondrocytes from being damaged during the injection phase, as the chondrocytes are maintained on the scaffold rather than suspended in collagen as seen in ACI and CACI [18].
A limitation of ACI is that the ex vivo growth of the chondrocytes may lead to loss of differentiation or loss of phenotype due to age-related decline in harvested chondrocytes, which renders them impotent for cartilage regeneration [20]. Although fetal and neonatal chondrocytes can develop significantly faster and with better collagen type II and proteoglycan structure, their limited availability has enabled a deeper investigation into other sources [18].
Pluripotent Stem Cells (PSCs)
Pluripotent stem cells (PSCs) are found to have the ability to differentiate into chondrocytes like embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) [18]. Even though ESCs are a phenomenal option for tissue regeneration, their unlimited potential to self-renew and differentiate into numerous tissue types raises ethical concerns as they are harvested from embryos in the blastocyst stage [18]. A superior alternative would be iPSCs, which can be generated from various cell types, including fibroblasts, cord blood cells, peripheral blood monocytes, and even a patient's skin or blood [18,21]. This provides a more ethical, less invasive, and reasonable option for PSCs to be harvested and used for cartilage regeneration. Further studies need to be conducted to evaluate the prevention of teratoma formation, as these undifferentiated cells can grow uncontrollably and are at risk for tumor formation [18].
Even though their potential for regenerative medicine and disease modeling has no ceiling, bioengineered grafts based on iPSCs are limited to only scientific and laboratory-scale productions [21]. Another limitation of iPSCs is the use of viral vectors for their generation, such as retro- or lentiviruses, which pose the possibility of genetic instability by integrating into the host cell genome or creating interference for host cell functions [21]. Several iPSC protocols have been studied in the past for chondrogenesis, but none have yet been approved for clinical trials. Thus, further studies are still required to further analyze controls and create a safe and stringent protocol for iPSC differentiation [21].
Mesenchymal Stem Cells (MSC)
MSCs, derived from adult tissue, carry a high proliferative capacity and capability to be multipotent, differentiating into adipocytes, chondrocytes, osteoblasts, and myocytes [18]. Another added benefit of using MSCs for chondrocyte regeneration is that they carry anti-inflammatory and regenerative characteristics, which help promote self-healing, while also producing collagen and proteoglycans, aiding in the growth and maintenance of cartilage [3]. The use of MSC and HA injected into the injured intra-articular site of porcine models showed cartilage regeneration [22]. However, it is important to acknowledge that although these cells display early clinical success, tissue repair commonly fails due to their negligible mechanical components [4].
The use of bone marrow stem cells (BMSCs), most commonly MSCs, has been associated with improved defect filling and higher collagen type II content following implantation in multiple animal studies. However, no clinical studies have yet been conducted to see if similar results can be replicated in humans [18].
Adipose-derived stem cells (ADSCs) are an alternative to BMSCs and are more plentiful and accessible, but do carry slightly lower chondrogenic potential [18]. Synovial-derived stem cells (SDSCs) have been associated with superior chondrogenic capacity when compared with other MSCs, such as skeletal muscle, adipose, bone marrow, and periosteum. Nonetheless, SDSCs are seen to withhold some fibroblastic capacity even after implantation, which deems them unreliable as a substitute for cartilage repair and regeneration [18].
The use of MSCs in the treatment of cartilage repair and regeneration has been promising, especially given their ability to differentiate into chondrocytes while also harboring anti-inflammatory mechanisms to promote healing and decrease rejection rates. Nevertheless, more thorough research is still required to understand the mechanism of signaling and optimal conditions for survival, proliferation, and MSC differentiation in vivo, while also needing strict protocols for administration methods and doses before they can be used in a broader clinical setting [3]. The use of autologous MSCs along with new tissue engineering techniques may hold the keys to becoming the panacea for cartilage repair and regeneration.
Scaffolds
The goal of employing scaffolds is to provide a temporary structure that establishes a three-dimensional design on which seeded cells can adhere, while also providing mechanical support that aids in cartilage development over time [18]. The scaffold material must possess specific characteristics: (i) controllable biodegradation and pliability without producing cytotoxic materials, (ii) the ability to allow nutrients and metabolite diffusion across the scaffold and regulate cell activity; (iii) support for cell proliferation, survival, differentiation and extracellular matrix production; (iv) the capacity to anchor into the cartilage defect site and integrate into the surrounding tissue; (v) provide mechanical stability and support for the new tissue; (vi) and appropriate regulation of surface chemistry to promote overall growth [18,21].
Scaffolds used for tissue engineering are broadly grouped into either natural or synthetic matrix materials [21]. Natural materials can be further categorized as protein or carbohydrate-based polymers, while composite polymers serve as a combination of natural and synthetic materials [18]. Several clinical studies have shown positive results from the use of natural or synthetic scaffolds for cartilage repair and regeneration, with most candidates claiming reduced pain and injury site healing compared to their counterparts, who received the traditional treatment of microfracture [18].
Techniques Based on Cellular Signaling
Cellular signaling using cytokines and growth factors is crucial in promoting the proliferation and growth of tissues and cartilage [21]. Chondrogenesis requires these endogenous molecules to signal in a coordinated autocrine and paracrine fashion to stimulate proper repair and regeneration [21]. The most common and studied cytokines involved in cartilage regeneration include the transforming growth factor (TGF) superfamily; platelet-derived growth factors (PDGF), insulin-like growth factor 1 (IGF-1), and fibroblast growth factors (FGF) [21].
Challenges of Generating Cartilage
One of the most challenging factors is due to the avascular nature of articular cartilage [17]. The lack of vascularization hinders progenitor cell penetration, thus limiting repair processes. Further, the restoration of osteochondral damage that includes subchondral bone is instituted by undifferentiated MSCs from bone marrow. The new tissue generated is comprised of fibrocartilage, which is an insufficient replacement for hyaline articular cartilage [17]. When considering the osteochondral grafting methods, injured osteochondral regions are replaced with autologous (mosaicplasty) or allogeneic (allograft transplantation) tissue [17]. There are concerns with donor site morbidity and graft failure during mosaicplasty, in addition to potential disease transmission and limited cell viability with allograft methods [17].
Huey et al. note that the greatest challenge in cartilage engineering is ensuring that the tissue can effectively perform one of its primary functions: bearing weight [4]. Currently, no biomedically generated materials on the market can fully replicate the compressive, tensile, and frictional properties of healthy cartilage on a large scale. Additionally, existing techniques fail to mimic the essential structure-function relationship of natural cartilage. When cartilage is loaded, the interstitial fluid within the joint becomes pressurized due to steric and electrostatic interactions, which hinder water movement and bear the majority of the load [4]. The remaining weight is then supported by the ECM. The complex ECM structure and tensile strength properties have been challenging to generate in a laboratory environment. However, advancements have been made with remodeling components, TGF-beta, as well as mechanical stimuli to improve the biomechanically engineered tensile forces [4]. A focus should be directed at augmenting collagen arrangement, maturation, and cross-linking [4].
The field of regenerative medicine has been continuously studying the various methods for cartilage regeneration to combat OA progression. Various approaches used currently to tackle cartilage repair include cell-based therapies, biologics, and physical therapy [3]. Tissue engineering is the future of cartilage regeneration, using biology and material science principles to promote tissue regeneration and repair [21]. This allows for potential biological and synthetic cartilage constructs to be implanted in a single step to promote durable tissue repair [18]. The advantages of tissue engineering lie in its ability to provide personalized treatment strategies while also promoting tissue repair and regeneration with minimal rejection and donor dependence [21]. The use of scaffold materials as the architectural support required for the cultured cells--harvested and expanded ex vivo for transplantation and stimulation of self-healing--is the core basis of this technology [21].
Conclusion
While medical and surgical methods have been the longstanding management for OA, cartilage regeneration provides promising results for future treatment of the disease. However, further research is still needed to fill the knowledge gaps before scaffolds and stem cells can be effectively combined for the successful creation of type II cartilage in previously injured osteoarthritic joints. The field of tissue engineering opens a gateway for durable tissue repair, incorporating stem cells and various scaffolds.
References
-
Centers for Disease Control and Prevention. Osteoarthritis. Available at https://www.cdc.gov/arthritis/osteoarthritis/index.html
-
Kolasinski, S. L., Neogi, T., Hochberg, M. C., Oatis, C., Guyatt, G., Block, J., Callahan, L., Copenhaver, C., Dodge, C., Felson, D., Gellar, K., Harvey, W. F., Hawker, G., Herzig, E., Kwoh, C. K., Nelson, A. E., Samuels, J., Scanzello, C., White, D., & Wise, B. (2020). 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care & Research, 72(2), 149–162. https://doi.org/10.1002/acr.24131
-
Cong, B., Sun, T., Zhao, Y., & Chen, M. (2023). Current and Novel Therapeutics for Articular Cartilage Repair and Regeneration. Therapeutics and clinical risk management, 19, 485–502. https://doi.org/10.2147/TCRM.S410277
-
Huey, D. J., Hu, J. C., & Athanasiou, K. A. (2012). Unlike bone, cartilage regeneration remains elusive. Science (New York, N.Y.), 338(6109), 917–921. https://doi.org/10.1126/science.1222454
-
Brody, L. T. (2015). Knee osteoarthritis: Clinical connections to articular cartilage structure and function. Physical Therapy in Sport, 16(4), 301–316. https://doi.org/10.1016/j.ptsp.2014.12.001
-
Buckwalter, J. A., Mankin, H. J., & Grodzinsky, A. J. (2005). Articular cartilage and osteoarthritis. Instructional course lectures, 54, 465–480.
-
Zhang, Y., & Jordan, J. M. (2010). Epidemiology of osteoarthritis. Clinics in geriatric medicine, 26(3), 355–369. https://doi.org/10.1016/j.cger.2010.03.001
-
Householder, N. A., Raghuram, A., Agyare, K., Thipaphay, S., & Zumwalt, M. (2023). A Review of Recent Innovations in Cartilage Regeneration Strategies for the Treatment of Primary Osteoarthritis of the Knee: Intra-articular Injections. Orthopaedic journal of sports medicine, 11(4), 23259671231155950. https://doi.org/10.1177/23259671231155950
-
Cirillo, D. J., Wallace, R. B., Wu, L., & Yood, R. A. (2006). Effect of hormone therapy on risk of hip and knee joint replacement in the Women's Health Initiative. Arthritis and rheumatism, 54(10), 3194–3204. https://doi.org/10.1002/art.22138
-
Nelson, A. E., Braga, L., Renner, J. B., Atashili, J., Woodard, J., Hochberg, M. C., Helmick, C. G., & Jordan, J. M. (2010). Characterization of individual radiographic features of hip osteoarthritis in African American and white women and men: The Johnston County Osteoarthritis Project. Arthritis Care & Research, NA-NA. https://doi.org/10.1002/acr.20067
-
Vaughn, I. A., Terry, E. L., Bartley, E. J., Schaefer, N., & Fillingim, R. B. (2019). Racial-Ethnic Differences in Osteoarthritis Pain and Disability: A Meta-Analysis. The journal of pain, 20(6), 629–644. https://doi.org/10.1016/j.jpain.2018.11.012
-
Callahan, L. F., Cleveland, R. J., Allen, K. D., & Golightly, Y. (2021). Racial/Ethnic, Socioeconomic, and Geographic Disparities in the Epidemiology of Knee and Hip Osteoarthritis. Rheumatic Diseases Clinics of North America, 47(1), 1–20. https://doi.org/10.1016/j.rdc.2020.09.001
-
McAlindon, T., LaValley, M., Schneider, E., Nuite, M., Lee, J. Y., Price, L. L., Lo, G., & Dawson-Hughes, B. (2013). Effect of Vitamin D Supplementation on Progression of Knee Pain and Cartilage Volume Loss in Patients With Symptomatic Osteoarthritis. JAMA, 309(2), 155. https://doi.org/10.1001/jama.2012.164487
-
Lane, N., Gore, L., Cummings, S., Hochberg, M., Scott, J., Williams, E., & Nevitt, M. (2001, March 22). Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: A longitudinal study [Review of Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: A longitudinal study]. Arthritis & Rheumatology; Wiley Online Library. https://onlinelibrary.wiley.com/doi/abs/10.1002/1529-0131(199905)42:5%3C854::AID-ANR3%3E3.0.CO;2-I
https://doi.org/10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-I -
Felson, D. T., Zhang, Y., Anthony, J. M., Naimark, A., & Anderson, J. J. (1992). Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Annals of internal medicine, 116(7), 535–539. https://doi.org/10.7326/0003-4819-116-7-535
-
Slemenda, C. (1997). Quadriceps Weakness and Osteoarthritis of the Knee. Annals of Internal Medicine, 127(2), 97. https://doi.org/10.7326/0003-4819-127-2-199707150-00001
-
Roseti, L., Desando, G., Cavallo, C., Petretta, M., & Grigolo, B. (2019). Articular Cartilage Regeneration in Osteoarthritis. Cells, 8(11). https://doi.org/10.3390/cells8111305
-
Tuan, R. S., Chen, A. F., & Klatt, B. A. (2013). Cartilage Regeneration. Journal of the American Academy of Orthopaedic Surgeons, 21(5), 303–311. https://doi.org/10.5435/jaaos-21-05-303
-
Brittberg, M., Lindahl, A., Nilsson, A., Ohlsson, C., Isaksson, O., & Peterson, L. (1994). Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. The New England journal of medicine, 331(14), 889–895. https://doi.org/10.1056/NEJM199410063311401
-
Adkisson, H. D., 4th, Martin, J. A., Amendola, R. L., Milliman, C., Mauch, K. A., Katwal, A. B., Seyedin, M., Amendola, A., Streeter, P. R., & Buckwalter, J. A. (2010). The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. The American journal of sports medicine, 38(7), 1324–1333. https://doi.org/10.1177/0363546510361950
-
Chen, M., Jiang, Z., Zou, X., You, X., Cai, Z., & Huang, J. (2024). Advancements in tissue engineering for articular cartilage regeneration. Heliyon, 10(3), e25400. https://doi.org/10.1016/j.heliyon.2024.e25400
-
Lee, K. B., Hui, J. H., Song, I. C., Ardany, L., & Lee, E. H. (2007). Injectable mesenchymal stem cell therapy for large cartilage defects--a porcine model. Stem cells (Dayton, Ohio), 25(11), 2964–2971. https://doi.org/10.1634/stemcells.2006-0311


A Modified Open Inguinal Hernia Repair Without Mesh Using a Fascia Turnover Flap in Low-Resource Settings
By: Cameron Rotbart
6.30.2025
Abstract
Introduction
Inguinal hernia repair is among the most common operations worldwide, with over 20 million procedures performed annually [1]. While mesh-based Lichtenstein repairs are the most common in high-resource settings, accessibility, cost, and complications limit their use in many low- and middle-income countries (LMICs) [2,3]. Additionally, synthetic mesh can cause chronic groin pain, infection, or erosion, specifically in areas with limited access to sterile surgical conditions or procedural follow-up [4,5].
Non-mesh techniques such as the Desarda and Bassini methods have resurfaced as practical alternatives in these specific contexts [6,7]. This paper presents a modification of a classic posterior wall reinforcement technique that utilizes a fascial turnover flap, derived from either the external oblique aponeurosis or transversalis fascia, to eliminate the need for mesh in open inguinal hernia repair.
Background
Synthetic mesh-based techniques, such as the Lichtenstein repair, are the standard for inguinal hernia management in high-resource settings due to their low recurrence rates [2]. However, in LMICs, access to sterile mesh remains inconsistent due to cost, supply chain limitations, and concerns about mesh-related complications such as chronic pain and infection [3,4]. There is a need for effective, reproducible, and cost-conscious alternatives when it comes to repairing one of the most common surgical procedures worldwide [1].
Inguinal hernia repair in LMICs faces unique challenges: high volume, limited access to mesh, and risk of infection. This technique adapts prior tissue-based methods by utilizing a turnover flap to reinforce the posterior wall without tension [6].
Desarda's method utilizes a muscle-aponeurotic strip of the external oblique [6]. This technique offers greater flexibility by harvesting from available fascia, and the modification provides mechanical support without relying on implants, thereby addressing both cost and supply chain issues [3,8].
The results show this approach is safe and effective in the short term, with minimal pain and no early recurrences. While randomized trials comparing this method to mesh repair are needed, these outcomes coincide with those from tissue repairs in similar populations [9,10].
Objective
To describe and evaluate a modified, mesh-free, fascia turnover flap technique for primary inguinal hernia repair as a viable alternative in low-resource environments.
Anatomy & Pathophysiology of Inguinal Hernias
Inguinal hernias occur at the inguinal canal, a natural weak point in the lower anterior abdominal wall where structures like the spermatic cord (in males) or the round ligament (in females) pass through the transversalis fascia. The canal is bound by the inguinal ligament inferiorly (the floor), the external oblique aponeurosis anteriorly (anterior wall), the transversalis fascia posteriorly (posterior wall), and is reinforced superiorly by the internal oblique and transversus abdominis muscles (the roof) [11].
Two primary forms of inguinal hernia exist:
Indirect inguinal hernias, which pass through the deep inguinal ring lateral to the inferior epigastric vessels, are often due to a patent processus vaginalis.
Direct inguinal hernias, which protrude medially to the epigastric vessels through Hesselbach’s triangle, which is commonly a zone of constitutional structural weakness.
The pathophysiology commonly involves increased intra-abdominal pressure combined with progressive weakening or disruption of the transversalis fascia, especially in elderly or physically active patients [12]. Chronic conditions like COPD, constipation, or heavy lifting can exacerbate this vulnerability [13].
Surgical Technique (In Males)
-
A standard 5–7 cm inguinal incision is made.
-
The external oblique aponeurosis is incised in the direction of its fibers to expose the inguinal canal [Figure 2].
-
The spermatic cord is isolated using a straight drain, and the hernia sac is identified [Figure 3].
-
In females, identify the round ligament of the uterus. This structure passes through the inguinal ring and can be ligated if needed.
-
In males, the spermatic cord must remain intact.
-
-
For indirect hernias, the sac is dissected free, reduced or excised, and ligated proximally.
-
For direct hernias, the transversalis fascia is plicated.
-
A 2 cm × 5–6 cm fascial flap is raised from either the lateral portion of the external oblique aponeurosis or transversalis fascia to preserve its vascular base.
-
The flap is turned medially and sutured to the inguinal ligament using interrupted 2-0 polypropylene sutures, which reinforce the posterior wall under minimal tension. Uninterrupted absorbable sutures may also be utilized [Figure 4,5].
-
In Females, the external oblique fascia flap is placed beneath the round ligament and follows a similar process as stated above.
-
-
The spermatic cord is returned to its anatomical position.
-
The external oblique is re-approximated over the cord, followed by skin closure [Figure 6].
-
No drains or mesh are used.
(Modified from techniques described in Desarda and Bassini principles [6,7]).

Figure 1. Step-by-step illustration of the modified open inguinal hernia repair without mesh using a fascial turnover flap. A. Skin incision and dissection of the inguinal canal. B. Isolation of the spermatic cord and hernia sac. C. Creation of a fascia turnover flap. D. Suture fixation of the flap to the inguinal ligament.
Illustration generated by ChatGPT with DALL·E 3, OpenAI (2025). The author confirms this image was created specifically for this publication and does not violate copyright.
Results
A total of 375 patients were included across two randomized clinical trials comparing Desarda and Lichtenstein repairs. Patients were predominantly male with primary, reducible inguinal hernias. The mean operative time ranged from 30 to 45 minutes, with no significant difference between techniques. Intraoperative complications were rare or absent.
At 3- to 6-month follow-up:
-
Recurrence rates were low and comparable between groups, ranging from 0% to 2.5%.
-
Chronic postoperative pain was significantly lower in the Desarda group, with one study reporting pain scores less than 3/10 on the Visual Analog Scale (VAS).
-
This VAS scale is used to assess pain, where no pain is ascribed a value of 0 and the most severe pain is ascribed a value of 10.
-
-
Patients undergoing Desarda repair resumed normal activities approximately one week earlier than those receiving mesh repair.
-
Wound infections and seromas were infrequent in both groups.
After reviewing the data, it was clear that the Desarda technique, by eliminating the need for mesh, reduces costs and mesh-related complications—barriers especially relevant in low- and middle-income countries [3,9,10].
Discussion
Assessing Advantages and Patient Suitability
This technique reinforces the posterior wall using autologous tissue to mimic tension-free mesh reinforcement, minimizing recurrence risk while avoiding foreign body implantation [4]. Advantages include lower cost, reduced risk of implant-related complications, and suitability for rural or low-resource hospitals. The repair mechanism preserves natural tissue planes and does not require prosthetic material [3,5].
When deciding which patients are better suited for this procedure, patients should have primarily reducible inguinal hernias, be from low-resource or mesh-unavailable environments, have a mesh allergy, or have prior mesh infection. Populations who are contraindicated in these procedures are those with recurrent hernias, large scrotal hernias, non-reducible or incarcerated hernias, or prior lower abdominal surgery with distorted or contorted anatomy [2,6].
Postoperative Management, Hazards, and Complications
Postoperative care mirrors that of traditional open hernia repairs. Patients are encouraged to ambulate within 12–24 hours. NSAIDs or acetaminophen typically manage pain and discomfort. Wound checks are done on days 5–7, with return to light activity by week 2, and full recovery by 4–6 weeks in most cases [1,3].
Potential complications include hematoma, seroma, flap necrosis, and nerve entrapment (especially ilioinguinal and iliohypogastric nerves). Flap ischemia can occur with excessive tension placed on the turnover segment or vascular compromise during dissection. The most concerning long-term risk is recurrence, often due to inadequate flap length or fixation to the inguinal ligament [4,5,12].
Limitations and Considerations
While the fascia turnover flap repair offers a promising solution for inguinal hernia repair in resource-constrained settings, several limitations and disadvantages warrant consideration. As stated above, it may not be suitable for large, recurrent, or incarcerated hernias where tissue is distorted or inadequate [6,10]. Additionally, there is a learning curve for flap creation, specifically when identifying and mobilizing the appropriate fascial plane. The technique is reliant on the surgeon's experience with tissue-based repair. While short-term results are promising, outcomes beyond 12 months are not yet well studied. Finally, while it avoids the risks associated with mesh, the durability and recurrence rates compared to standard Lichtenstein repair remain to be proven in randomized, controlled flap mobility trials. Until larger, controlled trials are available, this technique is best reserved for resource-constrained settings or specific patient populations [8,10].
Conclusion
The fascial turnover flap technique is a safe, cost-effective, and reproducible method for mesh-free inguinal hernia repair in low-resource settings. It avoids implant-related complications and provides acceptable short-term outcomes. Further research is required to confirm long-term durability and comparative efficacy.
Appendices:



Figure 6: External Oblique Aponeurosis closure in front of the cord is complete.
Acknowledgements:
Figures 2, 4-6, as shown in the appendices, were obtained and created by Desarda MP., 2008 [14] and utilized to depict Desarda’s new version of Non-Mesh Hernia Repair.
Figure 3 was obtained from Kapoor V., 2023 [15] to depict the surgical procedure step 3.
References
-
Beard JH, et al. Characterizing the global burden of surgical disease. World J Surg. 2016;40(1):132–138.
-
Kingsnorth AN, LeBlanc KA. Hernias: inguinal and incisional. Lancet. 2003;362(9395):1561–1571.
-
Grimes CE, Bowman KG, Dodgion CM, Lavy CB. Systematic review of barriers to surgical care in low-income and middle-income countries. World J Surg. 2011;35(5):941-950. doi:10.1007/s00268-011-1010-1
-
Nienhuijs S, Staal E, Strobbe L, Rosman C, Groenewoud H, Bleichrodt R. Chronic pain after mesh repair of inguinal hernia: a systematic review. Am J Surg. 2007;194(3):394–400. doi:10.1016/j.amjsurg.2007.02.012
-
Amid PK. Lichtenstein tension-free hernioplasty. Hernia. 2004;8(1):1–7.
-
Desarda MP. No-mesh inguinal hernia repair. Saudi J Gastroenterol. 2008;14(3):122–127.
-
Desarda MP. New method of inguinal hernia repair: a new solution. ANZ J Surg. 2001;71(4):241–244. doi:10.1046/j.1440-1622.2001.02092.x
-
Ghabisha S, Ahmed F, Ateik A. Inguinal hernia repair: a comparison of strengthening the posterior inguinal wall with aponeuroplasty versus the Lichtenstein technique (mesh repair). A randomized controlled trial in a low-resource setting. Arch Ital Urol Androl. 2025;97(2):115–121. doi:10.4081/aiua.2025.13790
-
Youssef T, El-Alfy K, Farid M. Randomized clinical trial of Desarda versus Lichtenstein repair for treatment of primary inguinal hernia. Int J Surg. 2015;20:28–34. doi:10.1016/j.ijsu.2015.05.055.
-
Szopinski J, Dabrowiecki S, Pierscinski S, Jackowski M, Jaworski M, Szuflet Z. Desarda versus Lichtenstein technique for primary inguinal hernia treatment: 3-year results of a randomized clinical trial. World J Surg. 2012;36(5):984-992. doi:10.1007/s00268-012-1508-1
-
Skandalakis JE, et al. Anatomy of the inguinal area. Am Surg. 2006;72(1):42–48.
-
Read RC. Attenuation of the transversalis fascia. Am J Surg. 1982;143(4):574–577.
-
Lau H, Lee F. Risk factors for inguinal hernia recurrence. Surg Endosc. 2005;19(6):784–788.
-
Desarda MP. No‑mesh inguinal hernia repair with continuous absorbable sutures: a dream or reality? (A study of 229 patients). Saudi J Gastroenterol. 2008;14(3):122–127. doi:10.4103/1319‑3767.41730
-
Kapoor V. Open Inguinal Hernia Repair Technique: approach considerations, Lichtenstein Tension-Free mesh Repair, other approaches. https://emedicine.medscape.com/article/1534281-technique#c2. Published 2023. Accessed June 19, 2025.
Achilles SpeedBridge Repair:
A Modern Alternative to Achilles Tendon Repair
By: Ryan Sholar, Archit Kumar
3.28.2025

Abstract
Introduction
Anatomy of the Achilles Tendon
The Achilles tendon is one of the strongest and most relied-upon structures in the human body. Connecting the Gastrocnemius and Soleus muscles of the calf to the Calcaneus of the heel, it is the primary plantar flexor of the ankle [1]. Almost any lower body movement such as walking, running, or jumping requires the actions of the Achilles tendon.
Mechanisms of Acute Achilles Tendon Ruptures
The Achilles tendon is most susceptible to rupture 3-6 cm proximal to its insertion point at the calcaneus [2]. Additionally, incidence rates are higher in the young male and geriatric male populations compared to other groups. The primary mechanisms causing the most stress to the tendon are pushing off with the forefoot while the knee is extended and sudden dorsiflexion of the ankle, especially if the motion is fierce while the foot is plantarflexed [2]. Since athletes repeatedly perform these movements and impose significant stress on the tendon during practice and competition, evidence has shown ruptures predominantly occur among this group.
Despite understanding the primary mechanisms behind the tendon rupturing, researchers and physicians are concerned with factors contributing to the deterioration of the Achilles tendon. Theories commonly considered include athletes returning to activity following an extended absence [2]. Upon returning to activity, the athlete’s technique may differ from how the ankle was typically used before their absence, which can cause unusual stress to the tendon. An example of this could include a football player returning from knee surgery who is in the beginning stages of walking and running again.
Pharmacological Contributions to Achilles Tendon Ruptures
Additionally, other theories causing degeneration of the Achilles tendon include pharmacological treatments such as quinolones and corticosteroids. A systematic review to determine the relationship between quinolone use and Achilles tendon ruptures indicated an increased risk with an odds ratio (OR) of 2.52, Confidence Interval (CI) of 1.81-3.52, and p < .001 [3]. Quinolones, in addition to being an effective antibiotic, reduce the transcription of decorin, a proteoglycan essential for maintaining the structural integrity of cartilage, skin, and tendons [4].
Corticosteroids, a commonly used anti-inflammatory medication, also contribute to tendon rupture due to impaired inflammatory and healing properties essential to maintaining structural integrity. Inflammatory cytokines released following injury to tendons stimulate tendon cell proliferation; when this process is impaired, tendons are damaged without restorative mechanisms for healing [5].
Standard Achilles Tendon Rupture Surgical Techniques
Surgical and percutaneous techniques exist for Achilles tendon rupture repair. Currently, the traditional surgical technique is the Open Achilles Tendon Repair technique in which the proximal and distal ends of the ruptured Achilles are sewn together utilizing a modified locking Bunnel stitch [6,7]. This technique should still be prioritized in patients who experience severe or chronic tendon damage, wide tendon ruptures, or have poor bone mineralization preventing sufficient bone anchoring with the speedbridge technique [6, 7, 8]
Figure 1: Open Achilles Tendon Repair technique utilizing a modified locking Bunnel stitch [9]
A percutaneous technique provides patients with an alternative to open surgery while providing adequate tendon repair. With the percutaneous technique, small incisions are made on the posterior calf, allowing physicians to insert instruments and materials to repair the damaged tendon internally utilizing the Arthrex PARS Guide, an instrument surgeons use to conduct their repair of the tendon [10]. Surgeons can also utilize ultrasound guidance while performing this technique.
Figure 2: Percutaneous Repair Technique via Arthrex PARS Guide [10]
SpeedBridge Technique as an Alternative
Alternative techniques for Achilles tendon repair are being explored due to current techniques requiring an extensive rehabilitation process for sometimes more than a year. One such technique is the SpeedBridge technique in which the damaged tendon is repaired using fiber tape anchored into the calcaneus [7]. In doing so, the tendon is repaired in a way that increases strength, stability, and durability when compared to the standard suturing technique [7]. As a result, patients can perform weight-bearing exercises earlier in the healing process, reducing the recovery duration [7]. Because of these advantages, the Speedbridge technique is being explored to become the modernized standard for Achilles tendon repairs.
Surgical Technique [7,8,11]
-
Place the patient in the prone position and apply a tourniquet to the proximal thigh
-
Make a 6-8 cm midline, longitudinal incision, exposing the paratenon, extending from the superior posterior edge of the calcaneus
-
Arrange the 2 tendon flaps laterally and anteriorly, exposing the Haglund's tubercle of the calcaneus
-
Using a saw and osteotome, remove Haglund's tubercle [Figure 3]
-
Approximately 1 cm proximal to the Achilles insertion point, drill a 4.75mm SwiveLock anchor into the lateral posterior calcaneus to the level of the shoulder stop on the drill guide
-
Insert the 4.75mm SwiveLock loaded with fiber tape into the anchor body until flush with the bone [Figure 4]
-
Repeat Steps 5 and 6 on the medial posterior surface of the calcaneus
-
Pass the needle attached to the fiber tape on the lateral side through the lateral tendon flap and pull the fiber tape through; repeat using the medial fiber tape, pulling it through the medial tendon flap [Figure 5]
-
Distal to the Achilles tendon insertion, drill a lateral and medial socket to the shoulder stop on the drill guide
-
Using one fiber tape tale from both the lateral and medial SwiveLock, adjust for tension, and insert the tails into the lateral socket until the anchor body is flush with the bone; repeat this step for the medial socket [Figure 6]
-
Place sutures along the paratenon and close the wound
Discussion
Achilles tendinopathy, rupture, Haglund's tubercle deformities, and other biomechanical vulnerabilities have burdened athletes, overweight populations, and aging individuals [13]. Specifically, the SpeedBridge technique has garnered interest due to its minimally invasive nature and better results for patients compared to traditional open Achilles repair and/or nonoperative alternatives [14].
Quicker Recovery Period
A retrospective study on 41 patients by Sattele et. al. showed that Percutaneous Achilles Repair System (PARS) with Midsubstance SpeedBridge allowed for a quicker recovery duration (119.2 +/- 44.0 days) compared to the traditional open surgical approach (169.4 +/- 41.6 days, P = 0.019) [15]. Furthermore, some studies claim the Speedbridge technique can further improve recovery times by incorporating an endoscopic approach versus open techniques. In a study with 89 patients, Lopes et al. found that those who underwent an endoscopic Speedbridge technique reported significantly better Victorian Institute of Sport Assessment - Achilles tendinopathy (VISA-A) questionnaire scores (p < 0.001) and European Foot & Ankle Society (EFAS) scores in both daily life (p < 0.001) and sports activity (p < 0.022) when assessed 3 months after procedure. They also found shoe discomfort at 6 months was less prominent in those who went through the endoscopic variant (3.7%, P=0.099) when compared to open surgery [16].
Improved Biomechanical Strength
A stress-loading experiment on 27 ankle cadavers by Melcher et. al. showed that the Achilles Midsubstance SpeedBridge repaired tendon elongated less than other common surgical interventions, PARS (P = 0.102), and the Dresdner instrument (P = 0.0006). The Midsubstance SpeedBridge cadavers also survived more cycles of cyclic loading from 20 to 100N (538 +/- 208) compared to the others in the study [17].
Reduced postoperative complications
Deng et. al. explored eight randomized controlled studies and 762 patients with an Achilles tendon rupture and concluded a 3.7% re-rupture rate in those following surgical intervention compared to a 9.8% re-rupture rate in nonoperative treatment (risk ratio 0.38, 95% confidence interval 0.21 to 0.68; p = .001) [14]. Post-operative complications such as infections, post-operative pain, lack of strength and mobility, deep venous thrombosis, and nerve damage pose limitations as innovations continue. A literature review by Traina et al. analyzing 465 procedures showed that the average complication rate in surgery treating Achilles tendinopathy was 18.3% [18]. Although limited in scope due to the novelty of the SpeedBridge technique, few studies such as those by Fradet et. al. and Swaroop et. al. have shown that minimally invasive approaches such as the endoscopic variant have greatly reduced the risk of post-surgery complications [9, 19].
Limitations and Future Direction
Current limitations of the SpeedBridge Achilles repair should be addressed as innovation continues. The most common complication of Achilles tendon surgery techniques, including the SpeedBridge, is suture cut out at the suture-tendon interface [17]. As with other forms of surgery, SpeedBridge complications include superficial wound complications, deep venous thrombosis, scar adhesion, infection, and sural nerve injury were evident in several study subjects undergoing the SpeedBridge technique [20]. Earlier elongation with the first few loading cycles post-surgery was seen in some patients compared to those who underwent other forms of treatment [16, 17]. Stringent postoperative management is indicated to ensure proper rehabilitation and stability of the repaired Achilles tendon. Different variations of the SpeedBridge can also be employed which can greatly alter results, intentionally or unintentionally. For example, endoscopic vs. open, single-row vs double-row, varying extents of debridement, different lengths and widths of FiberTape, allograft vs. autograft, knotless vs. knot, and other surgical decisions can be employed based on institution, expertise, or necessity [10, 21, 22, 23, 24, 25]. Age, race, gender, metabolic and genetic disorders, nutrition, extent of injury, postoperative management, and concomitant medications must also be addressed before considering a treatment approach [26].
Conclusion
The SpeedBridge technique is a novel surgery method to approach Achilles tendinopathies and ruptures. The method involves distinct intricacies that allow for quicker weight-bearing activities and stronger biomechanical forces to support the tendon and the foot. Although nonoperative treatments are available, studies have shown the risk of re-rupture, length of recovery, personal preference, lack of traditional recovery within 6 months, improved biomechanical foot strength, lower complications, and improvements in mechanical constructs have led to many opting for the SpeedBridge method. Patients and practitioners must adhere to the proper pre- and postoperative guidelines and protocols to ensure the best outcomes.
Appendices
Figure 4: Achilles Dissection with and without Haglund's Tubercle [10]
Figure 5: SwiveLock loaded with fiber tape [10]
Figure 6: Fiber tape passed through achilles tendon [10]
Figure 7: Resultant SpeedBridge suture technique [10,12]
References
-
O'Brien M. The anatomy of the Achilles tendon. Foot Ankle Clin. 2005 Jun;10(2):225-38. doi: 10.1016/j.fcl.2005.01.011. PMID: 15922915.
-
Kauwe M. Acute Achilles tendon rupture: Clinical evaluation, conservative management, and early active rehabilitation. Clin Podiatr Med Surg. 2017 Apr;34(2):229-243. doi: 10.1016/j.cpm.2016.10.009. Epub 2017 Jan 29. PMID: 28257676.
-
Alves C, Mendes D, Marques FB. Fluoroquinolones and the risk of tendon injury: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2019 Oct;75(10):1431-1443. doi: 10.1007/s00228-019-02713-1. Epub 2019 Jul 4. PMID: 31270563.
-
Chen S, Birk DE. Focus on Molecules: Decorin. Exp Eye Res. 2011;92(6):444-445. doi:10.1016/j.exer.2010.05.008.
-
Arvind V, Huang AH. Reparative and maladaptive inflammation in tendon healing. Front Bioeng Biotechnol. 2021;9:719047. doi:10.3389/fbioe.2021.719047.
-
Moore ML, Pollock JR, Karsen PJ, et al. Open Achilles tendon repair. JBJS Essent Surg Tech. 2023;13(1):e21.00054. doi:10.2106/JBJS.ST.21.00054.
-
Swaroop S, Dureja K, Vellaipandi V, Patnaik S. Speedbridge repair in degenerative achilles tear: A novel technique. Journal of Orthopaedic Case Reports. 2024;14(5):161-165. doi:10.13107/jocr.2024.v14.i05.4470
-
Hoffman J, Gupta S, Amesur A, Anthony T, Winder RP, Chan H, Hoang V. Achilles tendon rip-stop SpeedBridge repair. Arthroscopy Techniques. 2021;10(9):e2113-e2120. doi:10.1016/j.eats.2021.05.011
-
ClinicalPub. Open repair of Achilles tendon rupture. Available at: https://clinicalpub.com/open-repair-of-achilles-tendon-rupture/. Accessed March 20, 2025.
-
Waldron P, Kennedy JG, Guss D, et al. Percutaneous Achilles repair with high-strength suture tape augmentation: a technical note. Arthrosc Tech. Published online 2024. Available at: https://www.arthroscopytechniques.org/article/S2212-6287%2824%2900538-3/fulltext. Accessed March 20, 2025.
-
Achilles SpeedBridge Repair - Arthrex. YouTube. Published December 12, 2016. Accessed March 18, 2025. https://www.youtube.com/watch?v=BCvGTmTEZcM.
-
Arthrex. Achilles SpeedBridge System. Available at: https://www.arthrex.com/resources/VID1-0463-EN/achilles-speedbridge-system. Accessed March 20, 2025.
-
Lopes R, Ngbilo C, Padiolleau G, Boniface O. Endoscopic speed bridge: A new treatment for insertional achilles tendinopathy. Orthopaedics & Traumatology: Surgery & Research. 2021;107(6):102854. doi:10.1016/j.otsr.2021.102854
-
Deng S, Sun Z, Zhang C, Chen G, Li J. Surgical treatment versus conservative management for acute Achilles tendon rupture: A systematic review and meta-analysis of randomized controlled trials. The Journal of Foot and Ankle Surgery. 2017;56(6):1236-1243. doi:10.1053/j.jfas.2017.05.036
-
Sattele LA, Worts PR, Guyer AJ. A comparison of minimally invasive Achilles repair techniques on functional outcome and failure rates. American Orthopaedic Foot & Ankle Society. 2022;7(4):2473011421S00922. doi:10.1177/2473011421S00922
-
Clanton TO, Haytmanek CT, Williams BT, Civitarese DM, Turnbull TL, Massey MB, Wijdicks CA, LaPrade RF. A biomechanical comparison of an open repair and 3 minimally invasive percutaneous Achilles tendon repair techniques during a simulated, progressive rehabilitation protocol. American Journal of Sports Medicine. 2015;43(8):1957-1964. doi:10.1177/0363546515587082
-
Melcher C, Renner C, Piepenbrink M, Fischer N, Büttner A, Wegener V, Birkenmaier C, Jansson V, Wegener B. Biomechanical comparisons of three minimally invasive Achilles tendon percutaneous repair suture techniques. Clin Biomech (Bristol, Avon). 2022;92:105578. doi:10.1016/j.clinbiomech.2022.105578
-
Traina F, Perna F, Ruffilli A, Mazzotti A, Meliconi R, Berti L, Faldini C. Surgical treatment of insertional Achilles tendinopathy: a systematic review. J Biol Regul Homeost Agents. 2016;30(4 Suppl 1):131-138.
-
Fradet J, Lopes R. Endoscopic Calcaneal Speedbridge technique: Decreased postoperative complication rate in insertional achilles tendinopathy. Orthopaedics & Traumatology: Surgery & Research. 2024;110(5):103916. doi:10.1016/j.otsr.2024.103916
-
She G, Teng Q, Li J, Zheng X, Chen L, Hou H. Comparing surgical and conservative treatment on Achilles tendon rupture: a comprehensive meta-analysis of RCTs. Front Surg. 2021;8:607743. doi:10.3389/fsurg.2021.607743.
-
Lipman JD, Smith E, Rispoli DM, Orzechowski J, Sweeney K, Ho B, Patel M, Wapner KL. Jigless knotless internal brace versus other minimal invasive Achilles tendon repair techniques in biomechanical testing simulating the progressive rehabilitation protocol. J Foot Ankle Surg. 2022;61(4):761-766. doi:10.1053/j.jfas.2022.01.009
-
Nakajima K. Fluoroscopic and endoscopic calcaneal exostosis resection and Achilles tendon debridement for insertional Achilles tendinopathy: surgical techniques. Arthroscopy Techniques. 2023;12(6):e855-e860. doi:10.1016/j.eats.2023.02.018
-
Lopes R, Padiolleau G, Fradet J, Vieira TD. Endoscopic Speedbridge procedure for the treatment for insertional achilles tendinopathy: The snake technique. Arthroscopy Techniques. 2021;10(9). doi:10.1016/j.eats.2021.05.014
-
Ikuta Y, Nakasa T, Kawabata S, Adachi N. Achilles Tendon Reconstruction Using a Hamstring Tendon Autograft for Chronic Rupture of the Achilles Tendon in Patients Over 70 Years of Age: A Retrospective Case Series. Cureus. 2023;15(8):e42788. Published 2023 Aug 1. doi:10.7759/cureus.42788
-
Beitzel K, Mazzocca AD, Obopilwe E, Boyle JW, McWilliam J, Rincon L, Dhar Y, Arciero RA, Amendola A. Biomechanical properties of double- and single-row suture anchor repair for surgical treatment of insertional Achilles tendinopathy. American Journal of Sports Medicine. 2013;41(7):1642-1648. doi:10.1177/0363546513487061.
-
Sankova MV, Beeraka NM, Oganesyan MV, Rizaeva NA, Sankov AV, Shelestova OS, Bulygin KV, Vikram PRH, Barinov AN, Khalimova AK, Reddy YP, Basappa B, Nikolenko VN. Recent developments in Achilles tendon risk-analyzing rupture factors for enhanced injury prevention and clinical guidance: Current implications of regenerative medicine. Journal of Orthopaedic Translation. 2024;49:289-307. doi:10.1016/j.jot.2024.08.024.







